Automatic image segmentation of the abdominal aorta and stent grafts using artificial intelligence
Today’s technology for tomorrow’s medicine
by Bertram Sabrowsky-Hirsch, MSc und DI Dr. Stefan Thumfart
Aortic aneurysms are a critical vascular disease and are associated with thousands of deaths each year. The most common procedure performed is endovascular aortic repair (EVAR), in which a stent-graft prosthesis is placed in the abdominal aorta to prevent rupture. However, the procedure is associated with a high complication rate and requires regular follow-up examinations. These can now be improved by means of segmentation and calculation of measures.
Table of contents
- Reducing complications of endovascular aortic repair
- Innovative solution: Centreline-based method
- References
- Authors

Reducing complications of endovascular aortic repair
An abdominal aortic aneurysm is often discovered by chance, rarely manifesting itself with unclear symptoms such as abdominal pain or radiating pain in the back or groin. The frequency of an abdominal aneurysm in the population is around 40 per 100,000 people, mainly men over the age of 65. [1].
Once the diagnosis has been made, a decision must be made together with the doctor as to which treatment is appropriate. Currently, an “endovascular aortic repair” (EVAR) is chosen in 3/4 of cases, as it is less stressful than surgery. In EVAR, a stent-graft (Fig. 1) is inserted via a catheter in such a way that the aneurysm wall is relieved and thus the risk of rupture is eliminated. A disadvantage of the method, however, is that ongoing follow-up examinations are necessary, as complications (e.g. so-called endo-leaks) can occur.
In addition to the skills of the doctor and a high-quality stent-graft, the vascular structure is also important for the success of EVAR. Ideally, a CT-A scan (computer tomography – angiography, a 3D imaging technique that uses contrast to highlight the blood lumen) prior to the procedure could be used to decide how likely a complication is to occur or how often a follow-up examination is necessary. This is based on the automatic extraction and characterisation of the blood vessel and stent-graft from these scans.
The automatic extraction of the aorta and stent-graft must fulfil the following characteristics:
- fast runtime for clinical application on consumer hardware,
- robust for different stent-graft types and vessel topologies,
- High resolution for exact detection of the (fine) stent-graft lattice structures.

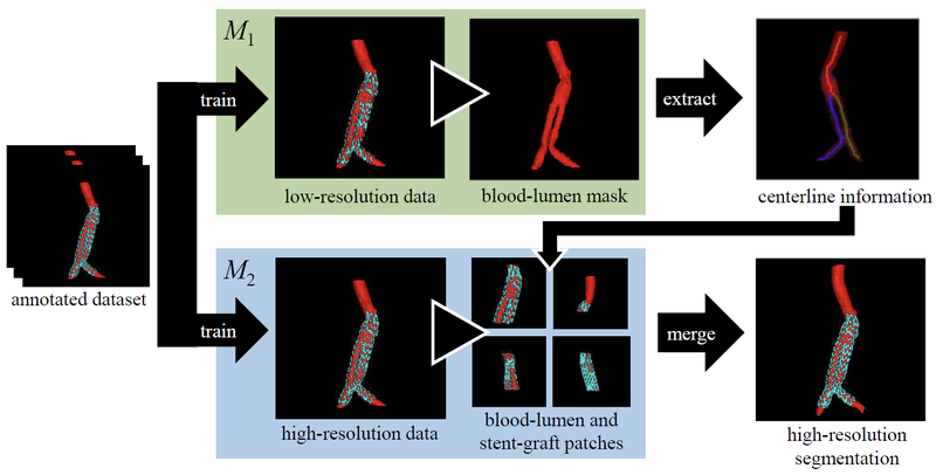
Fig.1 Stent.Graft
Source: https://sml.snl.no/stent
Fig. 2 Segmentation of aorta and stent graft in two phases. Top: Extraction of the centreline from a low-resolution scan. Bottom: Segmentation from high resolution scan patches. These patches are positioned using the centreline information.
Innovative solution: Centreline-based method
The solution developed by RISC Software GmbH [3, 4] is based on current methods for medical segmentation using Deep Learning. So-called U-nets [5] have become established as the basic model architecture in this field. Researchers have developed a two-phase approach (Fig. 2). First, the centrelines of the aorta are determined using a low-resolution data set. This can also be done in one step on consumer graphics cards with less texture memory. Based on these media lines, smaller (high-resolution) patches are extracted from the scan in phase 2 and used for training or prediction. In this way, even fine details (stent-graft grids) can be segmented reliably. The method was evaluated for 76 CT-A scans (36 patients). The average (median) DICE score is 0.969 (blood lumen) and 0.879 (stent-graft).
The centreline-based method can be trained and executed on a consumer graphics card (NVIDIA GTX 1080 TI). Compared to the state-of-the-art segmentation framework nnU-Net [6], the prediction time for a 3D scan could be reduced from an average of 10min 24sec to 1min 54sec – with the same accuracy. In total, scans with five different stent-graft types were successfully segmented.
As part of the research project EndoPredictor [2], RISC Software GmbH together with MATTES Medical Imaging and Kepler University Hospital MCIII investigated how well complications can be predicted. A scientific publication of the results is currently being prepared.
References
[1] https://www.gesundheit.gv.at/krankheiten/herz-kreislauf/arterien/aneurysma-aorta
[2] https://risc-software.at/endopredictor/
[3] Sabrowsky-Hirsch, Bertram, Stefan Thumfart, Richard Hofer, Wolfgang Fenz, Pierre Schmit, and Franz Fellner. “A Centerline-Guided Approach for Aorta and Stent-Graft Segmentation.” In Proceedings of the Joint Austrian Computer Vision and Robotics Workshop 2020, 102–7. Verlag der TU Graz, 2020. https://doi.org/10.3217/978-3-85125-752-6-24.
[4] Sabrowsky-Hirsch, Bertram, Stefan Thumfart, Wolfgang Fenz, and Richard Hofer. “Automatic Segmentation of the Abdominal Aorta and Stent-Grafts.” Journal of Image and Graphics, accepted 2021.
[5] Ronneberger, Olaf, Philipp Fischer, and Thomas Brox. “U-Net: Convolutional Networks for Biomedical Image Segmentation.” In Medical Image Computing and Computer-Assisted Intervention – MICCAI 2015, 234–41. Lecture Notes in Computer Science. Springer, Cham, 2015. https://doi.org/10.1007/978-3-319-24574-4_28.
[6] Isensee, Fabian, Paul F. Jaeger, Simon A. A. Kohl, Jens Petersen, and Klaus H. Maier-Hein. “NnU-Net: A Self-Configuring Method for Deep Learning-Based Biomedical Image Segmentation.” Nature Methods, December 7, 2020, 1–9. https://doi.org/10.1038/s41592-020-01008-z.
Authors

Bertram Sabrowsky-Hirsch, MSc
Researcher & Developer Medical Informatics

DI Dr. Stefan Thumfart
Project Manager & Senior Researcher